AG Alzheimer – Details
Publications
Full list of publications as listed in PubMed
Main Research Interests
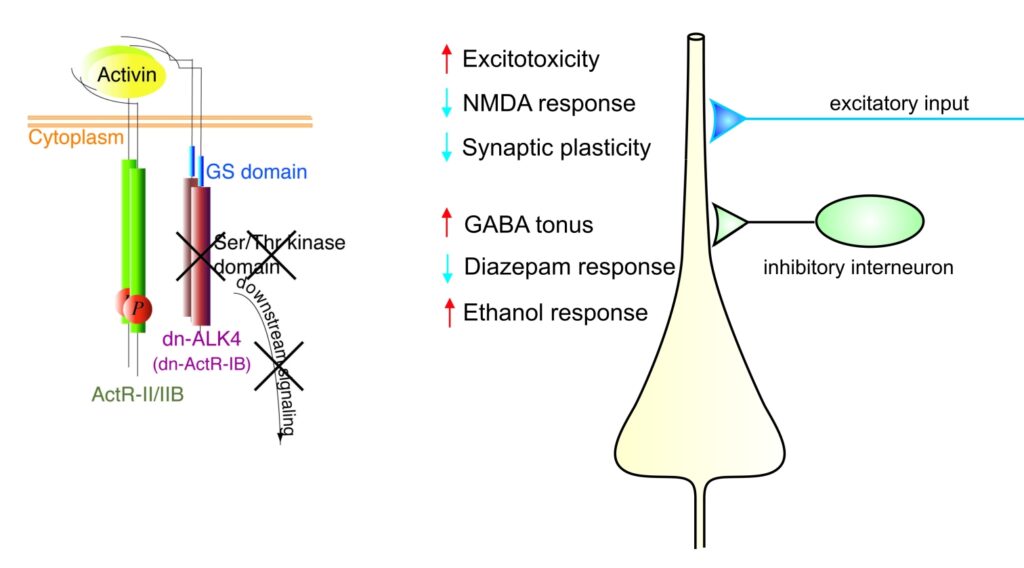
I. Role of Activin in Hippocampal Excitability and Plasticity, Anxiety-like Behavior, Depression, and Neuroprotection (group leader: Fang Zheng)
Activins belong to the transforming growth factor β (TGF β) superfamily and are now recognized as multifunctional regulatory proteins. Whereas we and others have firmly established a neuroprotective role of activin in acute brain injury, it remained unclear whether activin also influences the operation of neuronal circuits under physiological conditions. To elucidate the functions of activin in normal adult brain, S. Werner and her group at the Institute of Molecular Health Sciences (ETH Zurich) generated transgenic mice expressing a dominant-negative mutant of activin receptor IB (dnActRIB) under the control of the CaMKII-α promoter. The multiple effects of genetic disruption of activin receptor signaling on excitatory and inhibitory neurotransmission are summarized in the figure below. We are currently exploring how activin receptor signaling tunes cellular and network properties underlying cognitive functions and affective behavior.
Selected publications:
-
- Tretter YP, Hertel M, Munz B, ten Bruggencate G, Werner S and Alzheimer C: Induction of activin A is essential for the neuroprotective action of bFGF in vivo. Nature Med 6: 812-815, 2000.
- Müller M, Zheng F, Werner S and Alzheimer C. Transgenic mice expressing dominant-negative activin receptor IB in forebrain neurons reveal novel functions of activin at glutamatergic synapses. J Biol Chem 281:29076-29084, 2006.
- Zheng F, Adelsberger H, Müller MR, Fritschy J-M, Werner S and Alzheimer C: Activin tunes GABAergic neurotransmission and modulates anxiety-like behavior. Mol Psychiatry 14:332-346, 2009.
- Krieglstein K, Zheng F, Unsicker K and Alzheimer C. More than being protective: functional roles for TGF-beta/activin signaling pathways at central synapses. Trends Neurosci 34: 421-429, 2011.
- Link AS, Kurinna S, Havlicek S, Lehnert S, Reichel M, Kornhuber J, Winner B, Huth T, Zheng F, Werner S, Alzheimer C. Kdm6b and Pmepa1 as targets of bioelectrically and behaviorally induced activin A signaling. Mol Neurobiol 53:4210-4225, 2016.
- Zheng F, Puppel A, Huber SE, Link AS, Eulenburg V, van Brederode JF, Müller CP and Alzheimer C. Activin controls ethanol potentiation of inhibitory synaptic transmission through GABAA receptors and concomitant behavioral sedation. Neuropsychopharmacol 41: 2023-2044, 2016.
- Link AS, Zheng F and Alzheimer C. Activin signaling in the pathogenesis and therapy of neuropsychiatric diseases. Front Mol Neurosci 2016, doi: 10.3389/fnmol.2016.00032.
- Schmidt CC, Zheng F, Alzheimer C. Activin A regulates the excitability of hippocampal mossy cells. Hippocampus 32:401-410, 2022.
- Zheng F, Valero-Aracama MJ, Schaefer N, Alzheimer C. Activin A reduces GIRK current to excite dentate gyrus granule cells. Front Cell Neuroscience, doi.org/10.3389/fncel.2022.920388, 2022.
- Dahlmanns M, Jana Katharina Dahlmanns JK, Maria Jesus Valero-Aracama MJ, Zheng F, Alzheimer Environmental enrichment recruits activin A to recalibrate neural activity in mouse hippocampus. Cerebral Cortex 33: 663-675, 2023.
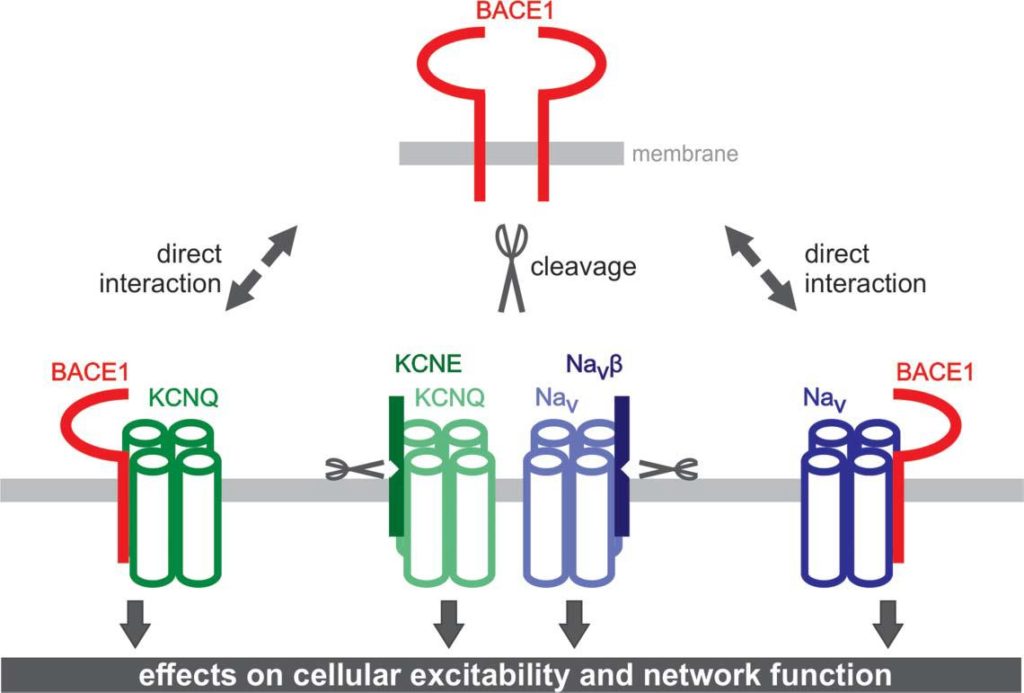
II. Properties and Functions of Na+ and K+ Channels and their regulation by BACE1. Physiological functions of BACE1 (group leader: Tobias Huth)
Using brain slices, acutely isolated neurons and heterologous expression systems, we are investigating properties and functions of voltage-dependent Na+ and K+ currents. In particular, we are studying how these currents are modulated by the β-site APP cleaving enzyme 1 (BACE1). In the context of these studies, we aim at identifying physiological functions of BACE1 besides its crucial role in the amyloid cascade hypothesis of Alzheimer´s disease.
Selected publications:
-
- Alzheimer C, Schwindt PC and Crill WE: Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat neocortex. J Neurosci 13: 660-673, 1993
- Lipowsky R, Gillessen T and Alzheimer C: Dendritic Na+ channels amplify EPSPs in hippocampal CA1 pyramidal cells. J Neurophysiol 76: 2181-2191, 1996.
- Takigawa T and Alzheimer C: G protein-activated inwardly rectifying K+ (GIRK) currents in dendrites of rat neocortical pyramidal cells. J Physiol (Lond.) 517:385-390, 1999.
- Huth T, Schmidt-Neuenfeldt K, Rittger A, Saftig P, Reiss K, Alzheimer C. Non-proteolytic effect of ß-site APP- cleaving enzyme 1 (BACE1) on sodium channel function. Neurobiol Dis 33: 282-289, 2009.
- Sittl R, Lampert A, Huth T, Schuy ET, Link AS, Fleckenstein J, Alzheimer C, Grafe P, Carr RW. Anti-cancer drug oxaliplatin induces acute cooling-aggravated neuropathy via Nav1.6-mediated resurgent and persistent current. Proc Natl Acad Sci (USA) 109:6704-6709, 2012.
- Hessler S, Zheng F, Hartmann S, Rittger A, Lehnert S, Völkel M, Nissen M, Edelmann E, Saftig P, Schwake M, Huth T, Alzheimer C. β-secretase BACE1 regulates hippocampal and reconstituted M-currents in a β-subunit-like fashion. J Neurosci 35: 3298-3311, 2015.
- Hartmann S, Zheng F, Kyncl M, Karch S, Voelkl K, Zott B, D’Avanzo C, Lomoio S, Tesco G, Kim DY*, Alzheimer C, Huth T. β-Secretase BACE1 promotes surface expression and function of Kv3.4 at hippocampal mossy fiber synapses. J Neurosci 38: 3480-3494, 2018
- Dierich M, Hartmann S, Dietrich N, Moeser P, Brede F, Johnson Chacko L, Tziridis K, Schilling A, Krauss P, Hessler S, Karch S, Schrott-Fischer A, Blumer M, Birchmeier C, Oliver D, Moser T, Schulze H, Alzheimer C, Leitner M, Huth T. β-secretase BACE1 is required for normal cochlear function. J Neurosci 39: 9013-9027, 2019
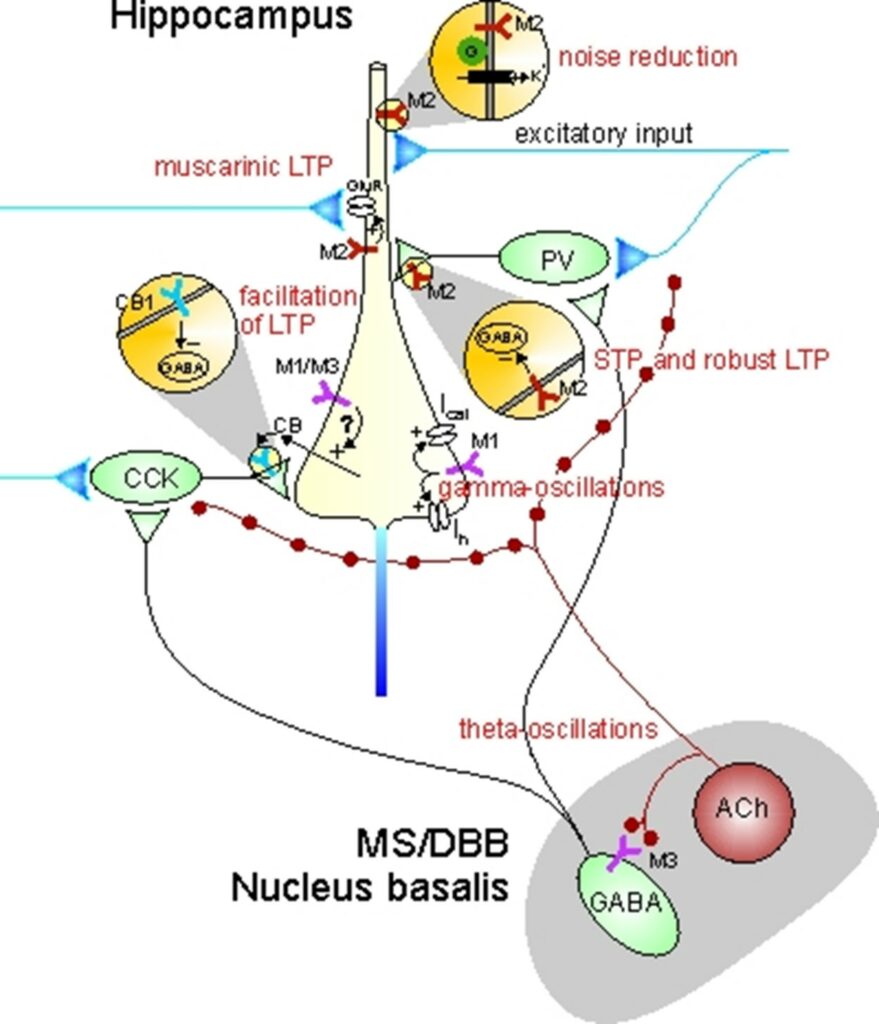
III. Neuropsychiatric disease models and mechanisms of drug action
Although muscarinic receptors are known to play central roles in facilitating cognitive functions, it is still not well understood how activation of individual receptor subtypes (M1 – M5) influences the neurobiological mechanisms that are thought to underlie learning and memory at the cellular and network level. Given the lack of muscarinic receptor ligands with a high degree of receptor subtype selectivity, we have used muscarinic receptor knock-out mice (in collaboration with Jürgen Wess, NIDDK, NIH, USA) to elucidate muscarinic effects on signal processing and synaptic plasticity. In collaboration with Carmen Villmann (Institute of Clinical Neurobiology, University of Würzburg), we have studied glycinergic neurotransmission in brainstem slices of a mouse model of startle disease. In collaboration with Christian Müller and Johannes Kornhuber (Dept. of Psychiatry, University Hospital Erlangen), we are exploring mechanisms of drug action in animal models of neuropsychiatric disease.
Selected publications:
-
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C and Wess J. M2 muscarinic acetylcholine receptor knockout mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci 24:10117-10127, 2004.
- Sydow A, van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou L, Rune G, Mandelkow E, D’Hooge R, Alzheimer C, Mandelkow EM. Tau-induced defects in synaptic plasticity, learning and memory are reversible in transgenic mice after switching off the toxic tau mutant. J Neurosci 31:2511-2525, 2011.
- Tischbirek CH, Wenzel EM, Zheng F, Huth T, Amato D, Trapp S, Denker A, Welzel O, Lueke K, Svetlitchny A, Rauh M, Deusser J, Schwab A, Rizzoli SO, Henkel AW, Müller CP, Alzheimer C, Kornhuber J, Groemer TW. Use-dependent inhibition of synaptic transmission by the secretion of intravesicularly accumulated antipsychotic drugs. Neuron 74:830-844, 2012.
- Schaefer N, Berger A, van Brederode J, Zheng F, Zhang Y, Leacock S, Littau L, Jablonka S, Malhotra S, Topf M, Winter F, Davydova D, Lynch J, Paige C, Alzheimer C, Harvey R, Villmann C. Disruption of a structurally important extracellular element in the glycine receptor leads to decreased synaptic integration and signaling resulting in severe startle disease. J Neurosci 37: 7948-7961, 2017.
- Amato D, Canneva F, Cumming P, Maschauer S, Groos D, Wrosch J, Groemer T, Chiofalo L, Dahlmanns M, Zheng F, Kornhuber J, Prante O, Alzheimer C, von Hörsten S, Müller CP. A dopaminergic mechanism of antipsychotic drug efficacy, failure, and failure reversal: the role of the dopamine transporter. Mol Psychiatry 25: 2101-2118, 2020
- Groos D, Zheng F, Rauh M, Quinger B, Kornhuber J, Müller CP, Alzheimer C. Chronic antipsychotic treatment targets GIRK current suppression, loss of LTD, and behavioral sensitization in a mouse model of amphetamine psychosis. J Psychopharmacol 33: 74-85, 2019
- El Hamdaoui Y, Zheng F, Fritz N, Ye L, Anh Tran M, Schwickert K, Schirmeister T, Braeuning A, Lichtenstein D, Hellmich UA, Weikert D, Heinrich M, Treccani G, Schäfer MKE, Nowak G, Nürnberg B, Alzheimer C, Müller CP, Friedland K. Analysis of hyperforin (St. John’s wort) action at TRPC6 channel leads to the development of a new class of antidepressant drugs. Mol Psychiatry org/10.1038/s41380-022-01804-3, 2022.
IV. Emergent Properties of Biological Neuronal Networks in Health and Disease (Junior Group Leader: Jana Dahlmanns)
Our research group is dedicated to unraveling the emergent properties of neuronal networks—complex behaviors and functions that arise from the intricate interplay of individual neurons with each other and with their microenvironments. By integrating approaches from molecular biology, imaging, and computational analyses, we aim to elucidate the fundamental mechanisms underlying learning, plasticity, and their perturbations in neurological diseases.
Network Synchrony and Connectivity: Utilizing live-cell calcium imaging combined with graph-theoretical analyses, we investigate how neuronal microcircuits achieve synchrony and adapt their connectivity patterns in response to various stimuli. Our studies have revealed that pharmacological agents, including antidepressants, can modulate network topology by reducing overall connectivity while enhancing modularity. These findings provide insights into how therapeutic interventions may promote network reorganization and functional recovery in psychiatric conditions.
Mitochondrial Dynamics and Metabolic Balance: We explore how mitochondrial morphology and function contribute to neuronal network stability and resilience. Our research indicates that signaling molecules like Activin A play a pivotal role in maintaining mitochondrial integrity, thereby preventing neuronal death under neurotoxic conditions. Additionally, we examine the intercellular transfer of mitochondria between neurons and glial cells, a process that may support metabolic balance and network homeostasis.
Glioblastoma and Ferroptosis: Extending our focus to neuropathology, we investigate the role of ferroptosis—a form of iron-dependent cell death characterized by lipid peroxidation—in glioblastoma progression and treatment. Our work assesses the genetic profiles associated with ferroptosis in malignant brain tumors and evaluates the potential off-target effects of ferroptosis induction on healthy neuronal tissues. These studies aim to inform the development of targeted therapies that minimize collateral damage to normal brain function.
By examining these interconnected aspects, our research seeks to provide a comprehensive understanding of how emergent properties of neuronal networks contribute to both the robustness of brain function and the vulnerabilities that lead to neurological disorders.
Selected publications:
- Yakubov, E., Schmid, S., Hammer, A., Chen, D., Dahlmanns, J. K., Mitrovic, I., … & Dahlmanns, M. (2023). Ferroptosis and PPAR-gamma in the limelight of brain tumors and edema. Frontiers in Oncology, 13, 1176038.
-
Dahlmanns, M., Dahlmanns, J. K., Savaskan, N., Steiner, H. H., & Yakubov, E. (2023). Glial glutamate transporter-mediated plasticity: system xc-/xCT/SLC7A11 and EAAT1/2 in brain diseases. Frontiers in Bioscience-Landmark, 28(3), 57.
-
Dahlmanns, J. K., & Dahlmanns, M. (2023). Network Reconstruction as a Novel High-Level Marker of Functional Neuronal Viability. In Cell Viability Assays: Methods and Protocols (pp. 47-63). New York, NY: Springer US.
-
Trepl, J., Dahlmanns, M., Kornhuber, J., Groemer, T. W., & Dahlmanns, J. K. (2022). Common network effect-patterns after monoamine reuptake inhibition in dissociated hippocampus cultures. Journal of Neural Transmission, 129(3), 261-275.
- Dahlmanns, M., & Dahlmanns, J. K. (2022). Synaptic Vesicle Pool Monitoring with Synapto-pHluorin. In Synaptic Vesicles: Methods and Protocols (pp. 181-192). New York, NY: Springer US.
-
Wrosch, J. K., Einem, V. V., Breininger, K., Dahlmanns, M., Maier, A., Kornhuber, J., & Groemer, T. W. (2017). Rewiring of neuronal networks during synaptic silencing. Scientific Reports, 7(1), 11724.