AG Ponomarenko – Details
Main Research Topics
I. Neuronal mechanisms, cognitive control and pathology of innate behaviors
The overarching goal of this research is to shed light on physiological as well as pathological real-time interactions between brain systems, supporting learned and innate behaviors. Innate behaviors are conserved across species and orchestrated primarily by the hypothalamus. Their examples include exploration of the environment, food seeking and intake, diverse social behaviors, avoidance of noxious stimuli and regulation of vigilance states. Appropriate initiation and termination of these crucial patterns relies on the availability of resources, environmental cues, hormonal regulations and previous experience. How is this information presented to and processed by the hypothalamus in order to generate adaptive and flexible behavioral outputs? A search for the answers to these questions has been fruitful for studies of neural circuits regulating innate behaviors, behavioral states and their disorders (e.g. sleep and feeding disorders, autism). Our highly interdisciplinary approach combines monitoring and manipulation of neuronal activity in behaving rodents with genetic and neuroanatomical methods as well as with advanced data analysis. Periodic changes of neuronal excitability have been a long-standing focus of research in the cortex and hippocampus where oscillations temporally organize collective activity of neuronal ensembles during encoding of sensory input and retrieval of memory traces.
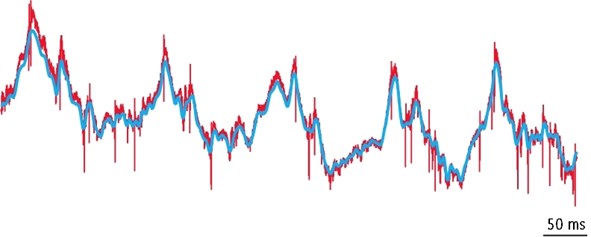
Much less is known about the influence of collective neuronal activity in associative brain regions on subcortical circuits directly generating behavioral output. Subcortical efferents of hippocampus most prominently innervate lateral septum (LS), which, in turn, is the main source of forebrain inputs to the hypothalamus. Using unitary and local field potential recordings in behaving mice we found that theta (5-10 Hz) and gamma (30-120 Hz) oscillations coordinate timing of neuronal discharge in LS with neuronal activity in the hippocampus and the medial prefrontal cortex (mPFC), respectively. We further found that gamma rhythms are coordinated between the LS and the lateral hypothalamus (LH), where firing probabilities during particular phases of gamma oscillations are related to a functional identity of neurons, defined by their activity during feeding behavior. These differential responses were found also in cells of the same neurochemical identity, as exemplified by LH VGAT-positive cells, showing strong gamma rhythmicity in vivo and gamma resonance ex vivo.
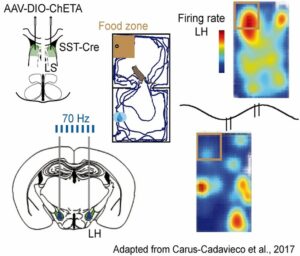
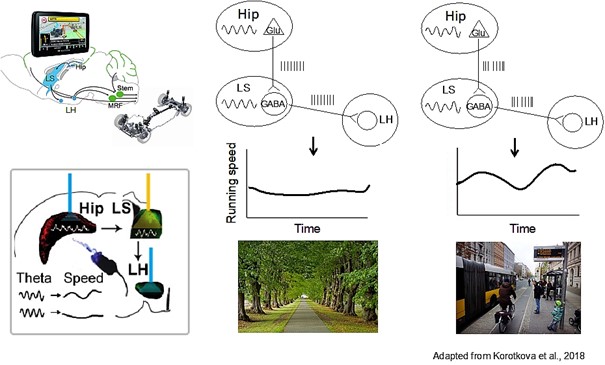
Using several optogenetic entrainment and inhibition approaches, we found that hippocampal theta oscillatory signaling via LS to LH regulate running speed, while gamma rhythmic activity of mPFC efferents support food seeking, during spontaneous behavior and in a cognitively demanding task.
Selected publications:
- Bender, F., Gorbati, M., Carus Cadavieco, M., Denisova, N., Gao, X., Holman, C., Korotkova, T., Ponomarenko, A. Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nature Communications, 2015; 6:8521 doi: 10.1038/ncomms9521.
- Carus Cadavieco, M., Gorbati, M., Ye, L., Bender, F., van der Veldt, S., Kosse, C., Börgers, C., Lee, S. Y., Ramakrishnan, C., Hu, Y., Denisova, N., Ramm, F., Volitaki, E., Burdakov, D., Deisseroth, K., Ponomarenko, A., Korotkova, T. Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking. Nature, 2017; 542(7640): 232-236.
- Herrera C.G., Ponomarenko A., Korotkova T., Burdakov D., Adamantidis A. Sleep & metabolism: The multitasking ability of lateral hypothalamic inhibitory circuitries. Front Neuroendocrinol., 2017; 44: 27-34.
- Gutierrez Herrera, C., Carus Cadavieco, M., Jego, S., Ponomarenko, A., Korotkova, T., Adamantidis, A. Hypothalamic feed-forward inhibition of thalamocortical network controls arousal and consciousness. Nature Neuroscience., 2016; 19(2): 290-8.
- Korotkova T., Ponomarenko A., Monaghan C.K., Poulter S.L., Cacucci F., Wills T., Hasselmo M.E., Lever C. Reconciling the different faces of hippocampal theta: The role of theta oscillations in cognitive, emotional and innate behaviors. Neurosci Biobehav Rev., 2018; 85: 65-80.
- Mederos S., Sánchez-Puelles C., Esparza J., Valero M., Ponomarenko A., Perea G. GABAergic signaling to astrocytes in the prefrontal cortex sustains goal-directed behaviors. Nat Neurosci., 2021; 24: 82-92.
II. Neural circuits synchronization and dysrhythmias
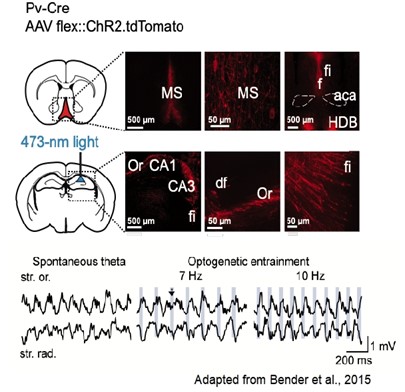
A perceivable reflection of brain dynamics, network oscillations are among most impressive brain signals. Thanks to their consistency and correlations with neuronal activity, oscillations offer a reliable physiological metric for studies of concurrent neuronal interactions in health and disease. First genetic mouse models of dysfunctional inhibition and excitation of interneurons in the laboratory of Hannah Monyer at the University of Heidelberg exhibited severe yet distinct alterations of hippocampal oscillations as well as of their coupling, partly reminiscent, also regarding behavioral phenotypes, of patients with dementia. More recently, working with the group of Thomas Jentsch at the FMP in Berlin, we investigated impaired network synchronization and spatial representations in the hippocampus upon the genetic ablation of KCNQ3- and dominant negative mutation of KCNQ5-potassium channels. Mutations of these channels have been linked to cognitive deficits in humans. Over the last years, we established a preparation for real-time optogenetic control of hippocampal theta oscillations in behaving mice and used it to further explore network mechanisms and functions of this rhythm, changes of which characterize several neuropsychiatric disorders.
Selected publications:
- Bender F, Korotkova T, Ponomarenko A. Optogenetic entrainment of hippocampal theta oscillations in behaving mice. J Vis Exp., 2018; 136. doi: 10.3791/57349.
- Fidzinski, P., Korotkova, T., Heidenreich, M., Maier, N., Schuetze, S., Kobler, O., Zuschratter, W., Schmitz, D., Ponomarenko, A., Jentsch, T.J. KCNQ5 K+ channels control hippocampal synaptic inhibition and fast network oscillations. Nature Communications, 2015; 6:6254 doi: 10.1038/ncomms7254.
- Korotkova, T., Fuchs, E., Ponomarenko, A., von Engelhardt, J., Monyer, H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representation and working memory. Neuron, 2010; 68(3):557-69.
- Ponomarenko, A.A., Korotkova, T.M., Sergeeva, O.A., Haas, H.L. Multiple GABAA receptor subtypes regulate hippocampal ripple oscillations. European Journal of Neuroscience, 2004; 20(8):2141-8.
- Stempel, A.V., Stumpf, A., Zhang, H.-Y., Özdogan, T., Pannasch, U., Theis, A-K., Otte, D-M., Wojtalla, A., Rácz, I., Ponomarenko, A., Xi, Z.-X., Zimmer, A., Schmitz, D. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron, 2016; 90(4):795-809.
- Racz, A., Ponomarenko, A., Fuchs, E., Monyer, H. Augmented hippocampal ripple-oscillations in mice with reduced fast excitation onto parvalbumin-positive cells. The Journal of Neuroscience, 2009; 29(8):2563-8.
- Wulff, P., Ponomarenko, A., Bartos, M., Korotkova, T., Fuchs, E., Bahner, F., Both, M., Tort, A., Kopell, N., Wisden, W., Monyer, H. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proceedings of the National Academy of Sciences, 2009; 106(9):3561-6.