Dr. Jana Katharina Dahlmanns
Dr. Jana Katharina Dahlmanns
Open Positions
Are you curious about how neurons work together to form complex networks—and how these networks adapt, protect, or fail in the face of disease?
We’re offering Bachelor and Master thesis positions in our group, where we explore the emergent properties of biological neuronal networks. Our research spans topics like:
🧠 neuronal network plasticity
🧬 mitochondrial dynamics and metabolic resilience
💊 drug effects on network connectivity
🧨 cell death mechanisms like ferroptosis in glioblastoma
You’ll gain hands-on experience in cutting-edge methods—from live-cell imaging to molecular biology—and be part of a small, collaborative team where curiosity and creativity are always welcome (and cake is occasionally mandatory 🍰).
🔬 Ideal for students in Molecular Medicine, Neuroscience, Medical Engineering or related fields.
🕒 Start date: flexible (preferably soon)
📩 Just drop me an email me at jana.dahlmanns@fau.de if you’re interested!
Let’s explore the brain—one network at a time.
Current projects:
– Mapping the Impact of Healthy and Epileptic Activity on Biological Neuronal Network Structure – best suited for M.Sc.
– Neuronal Survival Mechanisms: The Role of Activin A in Mitochondrial Dynamics – suited for B.Sc. or M.Sc.
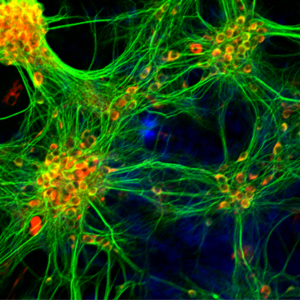
Research
Emergent Properties of Biological Neuronal Networks in Health and Disease (Junior Group Leader: Jana Dahlmanns)
Our research group is dedicated to unraveling the emergent properties of neuronal networks—complex behaviors and functions that arise from the intricate interplay of individual neurons with each other and with their microenvironments. By integrating approaches from molecular biology, imaging, and computational analyses, we aim to elucidate the fundamental mechanisms underlying learning, plasticity, and their perturbations in neurological diseases.
Network Synchrony and Connectivity: Utilizing live-cell calcium imaging combined with graph-theoretical analyses, we investigate how neuronal microcircuits achieve synchrony and adapt their connectivity patterns in response to various stimuli. Our studies have revealed that pharmacological agents, including antidepressants, can modulate network topology by reducing overall connectivity while enhancing modularity. These findings provide insights into how therapeutic interventions may promote network reorganization and functional recovery in psychiatric conditions.
Mitochondrial Dynamics and Metabolic Balance: We explore how mitochondrial morphology and function contribute to neuronal network stability and resilience. Our research indicates that signaling molecules like Activin A play a pivotal role in maintaining mitochondrial integrity, thereby preventing neuronal death under neurotoxic conditions. Additionally, we examine the intercellular transfer of mitochondria between neurons and glial cells, a process that may support metabolic balance and network homeostasis.
Glioblastoma and Ferroptosis: Extending our focus to neuropathology, we investigate the role of ferroptosis—a form of iron-dependent cell death characterized by lipid peroxidation—in glioblastoma progression and treatment. Our work assesses the genetic profiles associated with ferroptosis in malignant brain tumors and evaluates the potential off-target effects of ferroptosis induction on healthy neuronal tissues. These studies aim to inform the development of targeted therapies that minimize collateral damage to normal brain function.
By examining these interconnected aspects, our research seeks to provide a comprehensive understanding of how emergent properties of neuronal networks contribute to both the robustness of brain function and the vulnerabilities that lead to neurological disorders.
Selected publications:
- Yakubov, E., Schmid, S., Hammer, A., Chen, D., Dahlmanns, J. K., Mitrovic, I., … & Dahlmanns, M. (2023). Ferroptosis and PPAR-gamma in the limelight of brain tumors and edema. Frontiers in Oncology, 13, 1176038.
-
Dahlmanns, M., Dahlmanns, J. K., Savaskan, N., Steiner, H. H., & Yakubov, E. (2023). Glial glutamate transporter-mediated plasticity: system xc-/xCT/SLC7A11 and EAAT1/2 in brain diseases. Frontiers in Bioscience-Landmark, 28(3), 57.
-
Dahlmanns, J. K., & Dahlmanns, M. (2023). Network Reconstruction as a Novel High-Level Marker of Functional Neuronal Viability. In Cell Viability Assays: Methods and Protocols (pp. 47-63). New York, NY: Springer US.
-
Trepl, J., Dahlmanns, M., Kornhuber, J., Groemer, T. W., & Dahlmanns, J. K. (2022). Common network effect-patterns after monoamine reuptake inhibition in dissociated hippocampus cultures. Journal of Neural Transmission, 129(3), 261-275.
- Dahlmanns, M., & Dahlmanns, J. K. (2022). Synaptic Vesicle Pool Monitoring with Synapto-pHluorin. In Synaptic Vesicles: Methods and Protocols (pp. 181-192). New York, NY: Springer US.
-
Wrosch, J. K., Einem, V. V., Breininger, K., Dahlmanns, M., Maier, A., Kornhuber, J., & Groemer, T. W. (2017). Rewiring of neuronal networks during synaptic silencing. Scientific Reports, 7(1), 11724.
