Dr. Fang Zheng
Dr. Fang Zheng
Main Research Interests
I. Role of Activin in Hippocampal Excitability and Plasticity, Anxiety-like Behavior, Depression, and Neuroprotection (group leader: Fang Zheng)
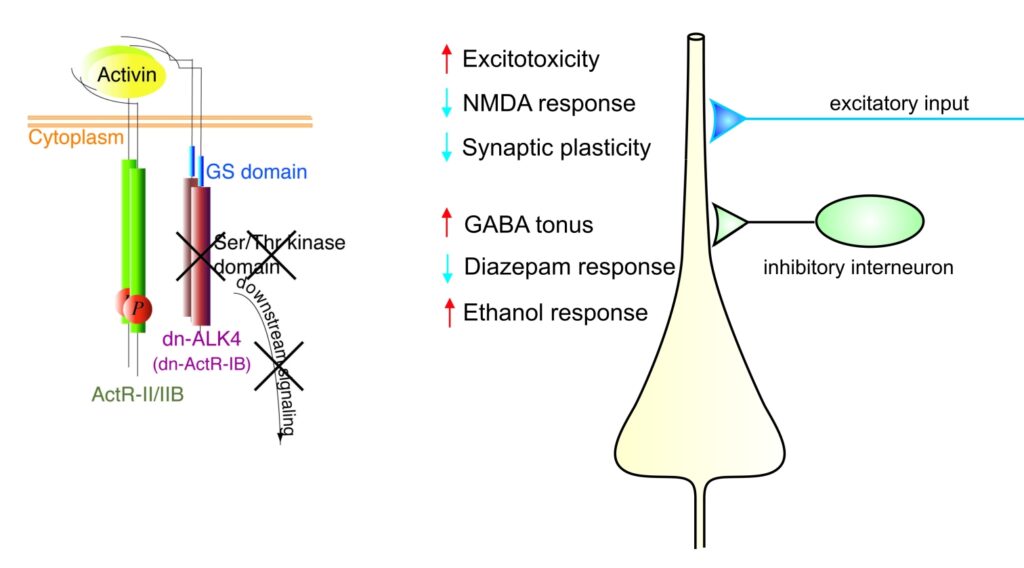
Activins belong to the transforming growth factor β (TGF β) superfamily and are now recognized as multifunctional regulatory proteins. Whereas we and others have firmly established a neuroprotective role of activin in acute brain injury, it remained unclear whether activin also influences the operation of neuronal circuits under physiological conditions. To elucidate the functions of activin in normal adult brain, S. Werner and her group at the Institute of Molecular Health Sciences (ETH Zurich) generated transgenic mice expressing a dominant-negative mutant of activin receptor IB (dnActRIB) under the control of the CaMKII-α promoter. The multiple effects of genetic disruption of activin receptor signaling on excitatory and inhibitory neurotransmission are summarized in the figure below. We are currently exploring how activin receptor signaling tunes cellular and network properties underlying cognitive functions and affective behavior.
Selected publications:
- Tretter YP, Hertel M, Munz B, ten Bruggencate G, Werner S and Alzheimer C: Induction of activin A is essential for the neuroprotective action of bFGF in vivo. Nature Med 6: 812-815, 2000.
- Müller M, Zheng F, Werner S and Alzheimer C. Transgenic mice expressing dominant-negative activin receptor IB in forebrain neurons reveal novel functions of activin at glutamatergic synapses. J Biol Chem 281:29076-29084, 2006.
- Zheng F, Adelsberger H, Müller MR, Fritschy J-M, Werner S and Alzheimer C: Activin tunes GABAergic neurotransmission and modulates anxiety-like behavior. Mol Psychiatry 14:332-346, 2009.
- Krieglstein K, Zheng F, Unsicker K and Alzheimer C. More than being protective: functional roles for TGF-beta/activin signaling pathways at central synapses. Trends Neurosci 34: 421-429, 2011.
- Link AS, Kurinna S, Havlicek S, Lehnert S, Reichel M, Kornhuber J, Winner B, Huth T, Zheng F, Werner S, Alzheimer C. Kdm6b and Pmepa1 as targets of bioelectrically and behaviorally induced activin A signaling. Mol Neurobiol 53:4210-4225, 2016.
II. Neuropsychiatric disease models and mechanisms of drug action
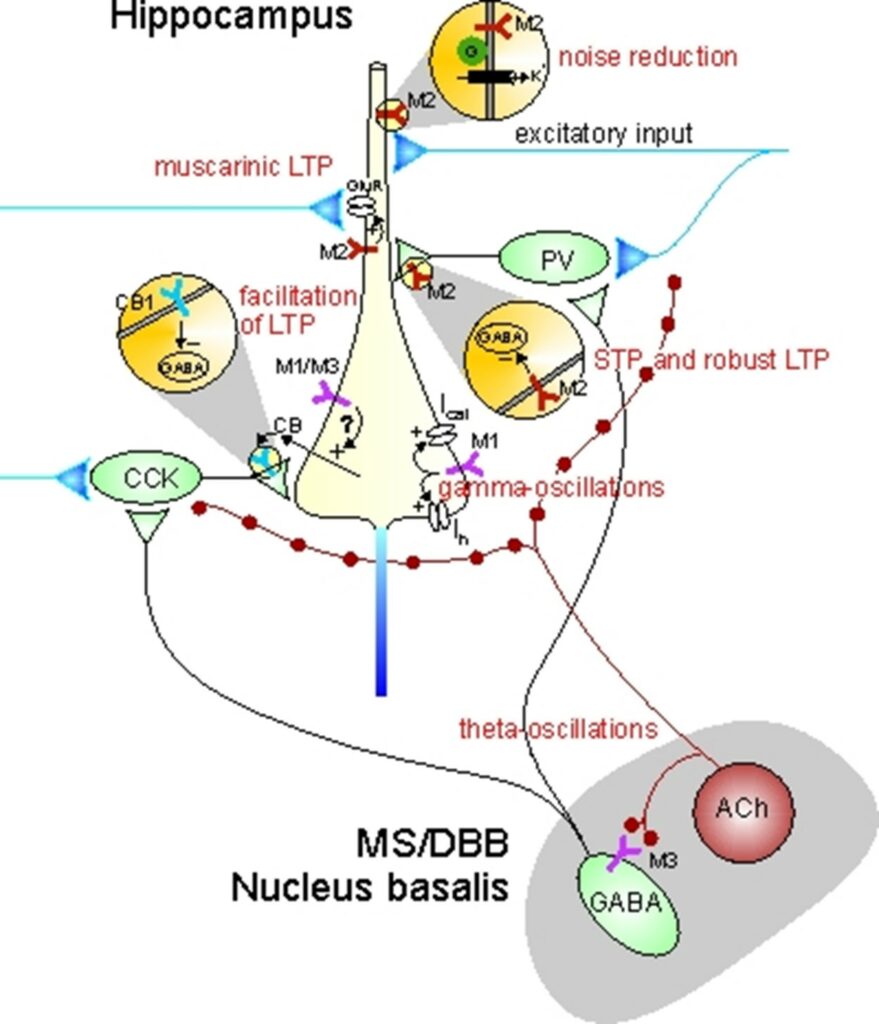
Although muscarinic receptors are known to play central roles in facilitating cognitive functions, it is still not well understood how activation of individual receptor subtypes (M1 – M5) influences the neurobiological mechanisms that are thought to underlie learning and memory at the cellular and network level. Given the lack of muscarinic receptor ligands with a high degree of receptor subtype selectivity, we have used muscarinic receptor knock-out mice (in collaboration with Jürgen Wess, NIDDK, NIH, USA) to elucidate muscarinic effects on signal processing and synaptic plasticity. In collaboration with Carmen Villmann (Institute of Clinical Neurobiology, University of Würzburg), we have studied glycinergic neurotransmission in brainstem slices of a mouse model of startle disease. In collaboration with Christian Müller and Johannes Kornhuber (Dept. of Psychiatry, University Hospital Erlangen), we are exploring mechanisms of drug action in animal models of neuropsychiatric disease.
Selected publications:
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C and Wess J. M2 muscarinic acetylcholine receptor knockout mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci 24:10117-10127, 2004.
- Sydow A, van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou L, Rune G, Mandelkow E, D’Hooge R, Alzheimer C, Mandelkow EM. Tau-induced defects in synaptic plasticity, learning and memory are reversible in transgenic mice after switching off the toxic tau mutant. J Neurosci 31:2511-2525, 2011.
- Tischbirek CH, Wenzel EM, Zheng F, Huth T, Amato D, Trapp S, Denker A, Welzel O, Lueke K, Svetlitchny A, Rauh M, Deusser J, Schwab A, Rizzoli SO, Henkel AW, Müller CP, Alzheimer C, Kornhuber J, Groemer TW. Use-dependent inhibition of synaptic transmission by the secretion of intravesicularly accumulated antipsychotic drugs. Neuron 74:830-844, 2012.
- Schaefer N, Berger A, van Brederode J, Zheng F, Zhang Y, Leacock S, Littau L, Jablonka S, Malhotra S, Topf M, Winter F, Davydova D, Lynch J, Paige C, Alzheimer C, Harvey R, Villmann C. Disruption of a structurally important extracellular element in the glycine receptor leads to decreased synaptic integration and signaling resulting in severe startle disease. J Neurosci 37: 7948-7961, 2017.
- Amato D, Canneva F, Cumming P, Maschauer S, Groos D, Wrosch J, Groemer T, Chiofalo L, Dahlmanns M, Zheng F, Kornhuber J, Prante O, Alzheimer C, von Hörsten S, Müller CP. A dopaminergic mechanism of antipsychotic drug efficacy, failure, and failure reversal: the role of the dopamine transporter. Mol Psychiatry 25: 2101-2118, 2020
- Groos D, Zheng F, Rauh M, Quinger B, Kornhuber J, Müller CP, Alzheimer C. Chronic antipsychotic treatment targets GIRK current suppression, loss of LTD, and behavioral sensitization in a mouse model of amphetamine psychosis. J Psychopharmacol 33: 74-85, 2019
- El Hamdaoui Y, Zheng F, Fritz N, Ye L, Anh Tran M, Schwickert K, Schirmeister T, Braeuning A, Lichtenstein D, Hellmich UA, Weikert D, Heinrich M, Treccani G, Schäfer MKE, Nowak G, Nürnberg B, Alzheimer C, Müller CP, Friedland K. Analysis of hyperforin (St. John’s wort) action at TRPC6 channel leads to the development of a new class of antidepressant drugs. Mol Psychiatry org/10.1038/s41380-022-01804-3, 2022.
